
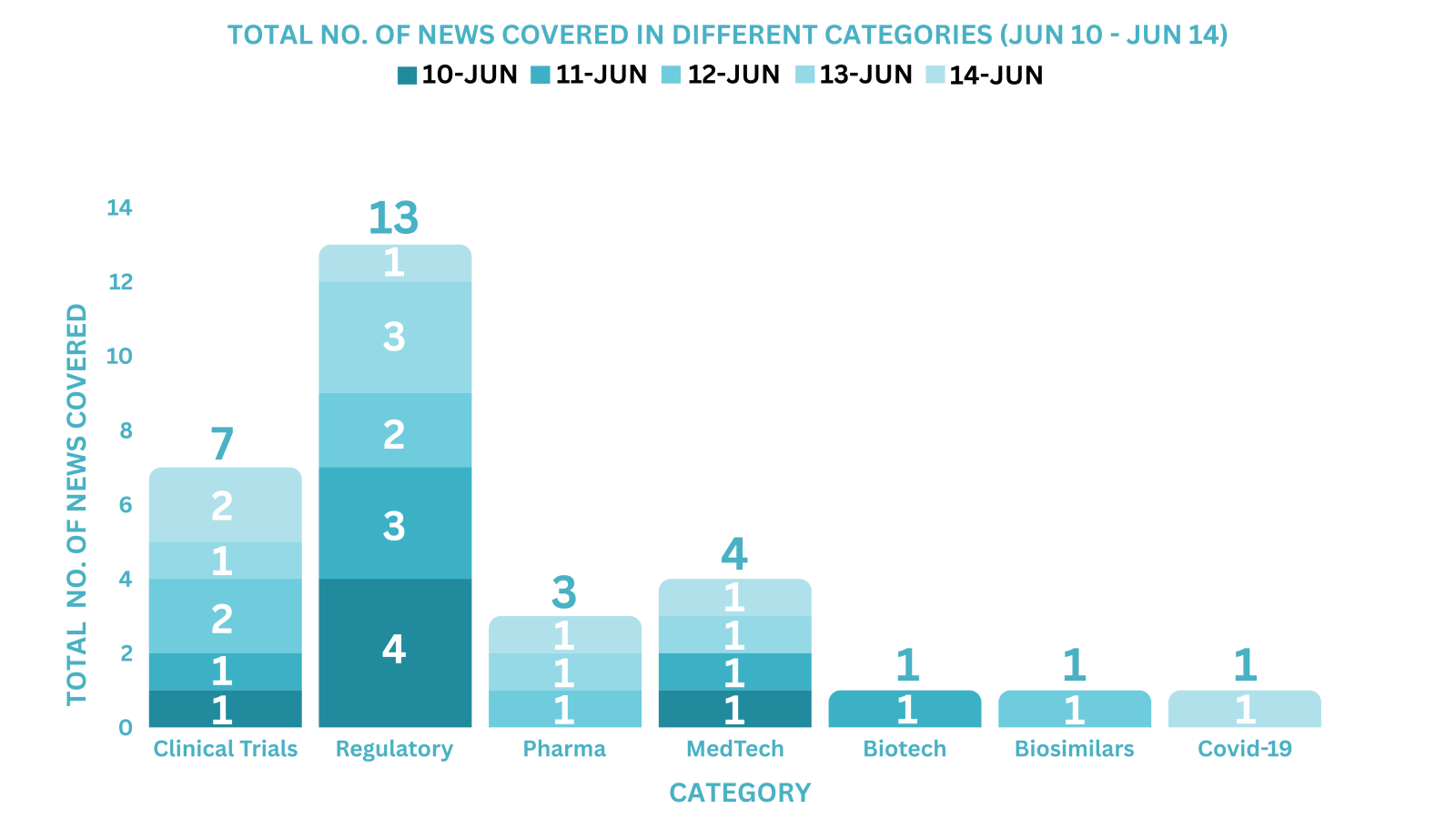
PharmaShots Weekly Snapshots (June 10 – June 14, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, COVID-19, Regulatory & MedTech. Check out our full report below:

Eli Lilly Reports the P-II (SYNERGY-NASH) Study Data of Tirzepatide for Treating Metabolic Dysfunction-Associated Steatohepatitis (MASH)
Read More: Eli Lilly
Moderna Reports the P-III Study Data of mRNA-1083 Vaccine Against Influenza and COVID-19
Read More: Moderna
Junshi Biosciences Reports the P-III Trial Data of Toripalimab Plus Bevacizumab to Treat Advanced Hepatocellular Carcinoma (HCC)
Read More: Junshi Biosciences
Palatin Technologies Reports the Commencement of P-II Trial Evaluating Bremelanotide to Treat Obesity
Read More: Palatin Technologies
Pfizer Reports the P-III Trial Data of Fordadistrogene Movaparvovec for Duchenne Muscular Dystrophy
Read More: Pfizer
BeiGene to Highlight Data from P-III (SEQUOIA) Study of Brukinsa in Combination with Venetoclax for CLL/SLL at EHA2024
Read More: BeiGene
Editas Medicine to Highlight the P-I/II/III (RUBY) Study Data of Reni-Cel for Treating Sickle Cell Disease at EHA 2024
Read More: Editas Medicine

Roche’s Alecensa (Alectinib) Receives the EC’s Approval as an Adjuvant Treatment for Early-Stage Lung Cancer
Read More: Roche
GSK’s Arexvy Gains the US FDA’s Approval for Expanded Age Indication to Prevent RSV Lower Respiratory Tract Disease
Read More: GSK
AstraZeneca Reports the US FDA’s sNDA Acceptance of Tagrisso to Treat Lung Cancer
Read More: AstraZeneca
Zymeworks Reports the NMPA’s BLA Acceptance of Zanidatamab to Treat Biliary Tract Cancer
Read More: Zymeworks
Ipsen’s Iqirvo (Elafibranor) Gains the US FDA’s Accelerated Approval for Treating Primary Biliary Cholangitis
Read More: Ipsen
The US FDA Grants Emergency Use Authorization to Roche’s Four-In-One Nucleic Acid Test
Read More: Roche
Regeneron’s Kevzara (Sarilumab) Gains the US FDA’s Approval to Treat Active Polyarticular Juvenile Idiopathic Arthritis (PJIA)
Read More: Regeneron
The Canadian Intellectual Property Office (CIPO) Issues Notice of Allowance for Patent Covering Palisade Bio’s PALI-2108 for Treating Ulcerative Colitis
Read More: Palisade Bio
60 Degrees Pharmaceuticals’ Tafenoquine Gains the US FDA’s Orphan Drug Designation to Treat Acute Babesiosis
Read More: 60 Degrees Pharmaceuticals
AstraZeneca’s Farxiga (Dapagliflozin) Bags the US FDA’s Approval to Treat Type-2 Diabetes in Pediatric Patients
Read More: AstraZeneca
LEO Pharma’s Adbry (tralokinumab-ldrm) Autoinjector Receives the US FDA’s Approval to Treat Moderate-to-Severe Atopic Dermatitis (AD)
Read More: LEO Pharma
Innovent’s IBI343 Gains the US FDA’s Fast Track Designation to Treat Pancreatic Cancer
Read More: Innovent
BMS Reports the US FDA’s Accelerated Approval of Augtyro to Treat Locally Advanced or Metastatic Solid Tumors
Read More: BMS

Flagship Pioneering and ProFound Therapeutics Team Up with Pfizer to Discover Novel Therapies to Treat Obesity
Read More: Flagship Pioneering & ProFound
Ochre Bio and GSK Team Up to Explore the Drivers of Liver Disease
Read More: Ochre Bio & GSK
AbbVie Teams Up FutureGen to Advance Next-Generation Therapy to Address Inflammatory Bowel Disease
Read More: Abbvie

DeepQure Reports the US FDA’s IDE Approval of HyperQure for Treating Resistant Hypertension
Read More: DeepQure
The US FDA Clears Abbott’s Two New Over-The-Counter Continuous Glucose Monitoring Systems
Read More: Abbott
Providence Medical’s CORUS Navigation Access System Gains the US FDA’s Clearance for Use in Posterior Spinal Fusion Procedures
Read More: Providence Medical
Venus Medtech Reports First Implantation of the VenusP-Valve in its Pivotal Trial Across the US
Read More: Venus Medtech

Mira Pharmaceuticals Reports Preclinical Data of Ketamir-2 to Treat Depression
Read More: Mira Pharmaceuticals

Alvotech and STADA Extend their Strategic Partnership Covering AVT03 (Biosimilar, Denosumab)
Read More: Alvotech & STADA

Moderna Reports the P-III Study Data of mRNA-1283 Vaccine to Prevent COVID-19
Read More: Moderna
Related Post: PharmaShots Weekly Snapshots (June 03 – June 07, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














